Abstract
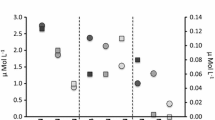
The Mediterranean endemic seagrass Posidonia oceanica is generally regarded as a stenohaline species, highly sensitive to salinity increments; however, in a few particular cases, natural populations can grow under salinity levels above its normal threshold of tolerance. One such case is a population of P. oceanica in the southeastern coastal region of Spain, which is able to survive under the fluctuating influence of hypersaline waters coming from an adjacent coastal lagoon (Mar Menor). The present work examines the physiological mechanisms underlying the species’ ability to overcome this hypersaline stress and persist in the long term. To this end, the physiological, morphological, and population statuses of plants from the site of influence were compared to those from a reference site where plants grew under normal conditions. P. oceanica leaves from the influenced sites showed more negative water potentials than those from the reference sites as a response to maintain a positive water balance under hypersaline conditions. However, these lower water potentials were not explained by the accumulation of intracellular solutes since their osmotic potential were similar to reference leaves and their ionic content generally lower. In addition, these leaves also accumulated higher concentrations of proline and soluble sugars but these organic osmolytes are more likely acting as osmo-protectants, rather than as osmoticums. These responses indicated that plants growing at the influenced site have developed physiological strategies to maintain lower ion concentration in their leaf tissues in order to avoid the alteration of ion homeostasis (i.e., ionic ratios), which can be toxic for plant metabolism. Photosynthesis and photochemistry were not adversely affected in leaves exposed to hypersalinity; in fact, these processes showed a tendency to become enhanced, possibly to support the assimilation of anthropogenic nitrogen coming from the lagoon waters. At the individual and population levels, P. oceanica plants growing under the influence of hypersaline waters exhibited a marked reduction in shoot size compared to those at the reference site, while shoot density and population growth rates were similar to those of the reference site and remained stable over time. This shoot size reduction involves a lower demand of metabolic resources necessary to maintain vegetative structures, which is mainly required for metabolic adjustments at the physiological level. We propose that this morphological adaptation serves as a stress-coping mechanism, helping the species to inhabit this unfavorable environment, as has been widely described for terrestrial plants subjected to long-term environmental stress.









Similar content being viewed by others
References
Alcoverro, T., C.M. Duarte, and J. Romero. 1995. Annual growth dynamics of Posidonia oceanica: contribution of large-scale versus local factors to seasonality. Marine Ecology Progress Series 120: 203–210.
Alcoverro, T., T. Manzanera, and J. Romero. 1998. Seasonal and age-dependent variability of Posidonia oceanica (L.) Delile photosynthetic parameters. Journal of Experimental Marine Biology and Ecology 230: 1–13.
Alcoverro, T., T. Manzanera, and J. Romero. 2001. Annual metabolic carbon balance of the seagrass Posidonia oceanica: the importance of carbohydrate reserves. Marine Ecology Progress Series 211: 105–116.
Arber, A. 1920. Water plants. A Study of Aquatic Angiosperms: Cambridge University Press, Cambridge.
Arevalo, L. 1988. El Mar Menor como sistema forzado por el Mediterráneo. Control hidráulico y agentes de fuerza. Boletín Instituto Español de Oceanografía 5: 63–96.
Bates, L. 1973. Rapid determination of free proline for water-stress studies. Plant and Soil 39: 205–207.
Biebl, R., and C.P. McRoy. 1971. Plasmatic resistance and rate of respiration and photosynthesis of Zostera marina at different salinities and temperatures. Marine Biology 8: 48–56.
Bisson, M.A., and G.O. Kirst. 1995. Osmotic acclimation and turgor pressure regulation in algae. Naturwissenschaften 82: 461–471.
Boudouresque, F.C., G. Bernard, G. Pegent, A. Shili, and M. Verlaque. 2009. Regression of Mediterranean seagrasses caused by natural processes and anthropogenic disturbances and stress: a critical review. Botanica Marina 52: 395–418.
Colmer, T.D., W.M. Teresa, F.A. Läuchli, and R.M. Higashi. 1996. Interactive effects of salinity, nitrogen and sulphur on the organic solutes in Spartina alterniflora leaf blades. Journal of Experimental Botany 47: 369–375.
Cosgrove, D.J. 1993. How do plant cell walls extend? Plant Physiology 102: 1–6.
Cramer, G.R. 2002. Sodium-calcium interactions under salinity stress. In Salinity: Environment e plants e molecules, ed. A. Läuchli and U. Lüttge, 205–229. Dordrecht: Kluwer Academic Publishers.
Crowder, M.J., and D.J. Hand. 1990. Analysis of repeated measures. New York: Chapman & Hall.
Dennison, W.C. 1990. Leaf production. In Seagrass research methods, ed. R.C. Phillips and P. McRoy. Paris: Unesco 81-85 pp.
Dodd, R.S., and V. Douhovnikoff. 2016. Adjusting to global change through clonal growth and epigenetic variation. Frontiers in Ecology and Evolution 4: 86. doi:10.3389/fevo.2016.00086.
Dreizin, Y., A. Tenne, and D. Hoffman. 2007. Integrating large scale seawater desalination plants within Israel’s water supply system. Desalination 220: 132–149.
Fernández-Torquemada, Y., and J.L. Sánchez-Lizaso. 2005. Effects of salinity on leaf growth and survival of the Mediterranean seagrass Posidonia oceanica (L.) Delile. Journal of Experimental Marine Biology and Ecology 320: 57–63.
Fernández-Torquemada, Y.F., J.L. Sánchez-Lizaso, and J.M.G. González-Correa. 2005. Preliminary results of the monitoring of the brine discharge produced by the SWRO desalination plant of Alicante (SE Spain). Desalination 182: 579–590.
Fourqurean, J.W., J.N. Boyer, M.J. Durako, L.N. Hefty, and B.J. Peterson. 2003. Forecasting responses of seagrass distributions to changing water quality using monitoring data. Ecological Applications 13: 474–489.
Fry, B., A. Gace, and J.V. McClelland. 2003. Chemical indicators of anthropogenic nitrogen loading in four Pacific estuaries. Pacific Science 57: 77–101.
Gacia, E., O. Invers, M. Manzanera, E. Ballesteros, and J. Romero. 2007. Impact of the brine from a desalination plant on a shallow seagrass (Posidonia oceanica) meadow. Estuarine Coastal and Shelf Science 72: 579–590.
Garrote-Moreno, A., J.M. Sandoval-Gil, J.M. Ruiz, L. Marín-Guirao, J. Bernardeau-Esteller, R. García, and J.L. Sánchez-Lizaso. 2015a. Plant water relations and ion homoeostasis of Mediterranean seagrasses (Posidonia oceanica and Cymodocea nodosa) in response to hypersaline stress. Marine Biology 162 (1): 55–68.
Garrote-Moreno, A., Y. Fernández-Torquemada, and J.L. Sánchez-Lizaso. 2014. Salinity fluctuation of the brine discharge affects growth and survival of the seagrass Cymodocea nodosa. Marine Pollution Bulletin 81 (1): 61–68.
Garrote-Moreno, A., A. McDonald, T.D. Sherman, J.L. Sánchez-Lizaso, K.L. Heck, and J. Cebrian. 2015b. Short-term impacts of salinity pulses on ionic ratios of the seagrasses Thalassia testudinum and Halodule wrightii. Aquatic Botany 120: 315–321.
Greenway, H., and R. Munns. 1980. Mechanisms of salt tolerance in non halophytes. Annual Review of Plant Physiology 31: 149–190.
Hasegawa, P.M., R.A. Bressan, J.K. Zhu, and H.J. Bohnert. 2000. Plant cellular and molecular responses to high salinity. Annual Review of Plant Physiology and Plant Molecular Biology 51: 463–499.
Hessini, K., J.P. Martínez, M. Gandour, A. Albouchi, A. Soltani, and C. Abdelly. 2009. Effect of water stress on growth, osmotic adjustment, cell wall elasticity and water-use efficiency in Spartina alterniflora. Environmental and Experimental Botany 67: 312–319.
Invers, O., G.P. Kraemer, M. Pérez, and J. Romero. 2004. Effects of nitrogen addition on nitrogen metabolism and carbon reserves in the temperate seagrass Posidonia oceanica. Journal of Experimental Marine Biology and Ecology 303: 97–114.
Iyer, V., and A.D. Barnabas. 1993. Effects of varying salinity on leaves of Zostera capensis Setchell. I. Ultrastructural changes. Aquatic Botany 46: 141–153.
Jagels, R. 1983. Further evidence for osmoregulation in epidermal leaf cells of seagrasses. American Journal of Botany 70: 327–333.
Kahn, A.E., and M.J. Durako. 2006. Thalassia testudinum seedling responses to changes in salinity and nitrogen levels. Journal of Experimental Marine Biology and Ecology 335: 1–12.
Kaya, C., and D. Higgs. 2003. Supplementary potassium nitrate improves salt tolerance in bell pepper plants. Journal of Plant Nutrition 26: 1367–1382.
Kerr, E.A., and S. Strother. 1985. Effects of irradiance, temperature and salinity on photosynthesis of Zostera muelleri. Aquatic Botany 23: 177–183.
Kirst, G.O. 1989. Salinity tolerance of eukaryotic marine algae. Annual Review of Plant Physiology and Plant Molecular Biology 40: 21–53.
Koch, M.S., S.A. Schopmeyer, C. Kyhn-Hansen, C.J. Madden, and J.S. Peters. 2007. Tropical seagrass species tolerance to hypersalinity stress. Aquatic Botany 86: 14–24.
Kuo, J., and C. Den Hartog. 2000. Seagrasses: a profile of an ecological group. Biologia Marina Mediterranea 7: 3–17.
Lambers, H., F.S. Chapin, and T.L. Pons. 2006. Plant physiological ecology. New York: Springer-Verlag.
Larkum, A.W.D., E.A. Drew, and P.J. Ralph. 2006. Photosynthesis and metabolism in seagrasses at the cellular level. In Seagrasses: Biology, ecology and conservation, ed. A.W.D. Larkum, R.J. Orth, and C.M. Duarte, 323–345. The Netherlands: Springer.
Latzel, V., A.P. Rendina Gonzalez, and J. Rosenthal. 2016. Epigenetic memory as a basis for intelligent behavior in clonal plants. Frontiers in Plant Science 7: 1354. doi:10.3389/fpls.2016.01354.
Lee, K.-S., S.R. Park, and Y.K. Kim. 2007. Effects of irradiance, temperature, and nutrients on growth dynamics of seagrasses: a review. Journal of Experimental Marine Biology and Ecology 350: 144–175.
Lichtenthaler, H.K. 1996. Vegetation stress: an introduction to the stress concept in plants. Plant Physiology 148: 4–14.
Lichtenthaler, H.K., and A.R. Wellburn. 1983. Determination of total carotenoids and chlorophyll a and b of leaf extracts in different solvents. Biochemical Society Transactions 603: 591–592.
Marín-Guirao, L., J. Lloret, and A. Marín. 2008. Carbon and nitrogen stable isotopes and metal concentration in food webs from a mining-impacted coastal lagoon. Science of the Total Environment 393: 118–130.
Marín-Guirao, L., J.M. Sandoval-Gil, J.M. Ruiz, and J.L. Sánchez-Lizaso. 2011. Photosynthesis, growth and survival of the Mediterranean seagrass Posidonia oceanica in response to simulated salinity increases in a laboratory mesocosm system. Estuarine, Coastal and Shelf Science 92: 286–296.
Marín-Guirao, L., J.M. Sandoval-Gil, J. Bernardeau-Esteller, J.M. Ruiz, and J.L. Sánchez-Lizaso. 2013a. Responses of the Mediterranean seagrass Posidonia oceanica to hypersaline stress duration and recovery. Marine Environmental Research 84: 60–75.
Marín-Guirao, L., J.M. Ruiz, J.M. Sandoval-Gil, J. Bernardeau-Esteller, C.M. Stinco, and A.J. Meléndez-Martínez. 2013b. Xanthophyll cycle-related photoprotective mechanism in the Mediterranean seagrasses Posidonia oceanica and Cymodocea nodosa under normal and stressful hypersaline conditions. Aquatic Botany 109: 14–24.
Mas, J. 1994. El Mar Menor: Relaciones, diferencias y afinidades entre la Laguna costera y el Mar Mediterráneo adyacente. PhD thesis, Autonomous University of Madrid.
Maxwell, K., and G.N. Johnson. 2000. Chlorophyll fluorescence—a practical guide. Journal of Experimental Botany 345: 659–668.
McClelland, J.W., and I. Valiela. 1998. Linking nitrogen in estuarine producers to land-derived sources. Limnology and Oceanography 43: 577–585.
McMillan, C., and F.N. Moseley. 1967. Salinity tolerances of five marine spermatophytes of Redfish Bay, Texas. Ecology 48: 503–506.
Munns, R. 2002. Comparative physiology of salt and water stress. Plant, Cell and Environment 25: 239–250.
Murphy, L.R., S.T. Kinsey, and M.J. Durako. 2003. Physiological effects of short-term salinity changes on Ruppia maritima. Aquatic Botany 75: 293–309.
Navarro G, Prieto L & Ruiz J. 2007. Estudio de la dinámica de circulación de la Manga del Mar Menor. Informe parcial de los resultados obtenidosen la campaña oceanográfica en el Mar Menor. Universidad de Granada y CEAMA-UGR, Granada, Spain, 150 pp.
Niu, X., R.A. Bressan, P.M. Hasegawa, and J.M. Pardo. 1995. Ion homeostasis in NaCl stress environments. Plant Physiology 109: 735–742.
Ogata, E., and T. Matsui. 1964. Photosynthesis in several marine plants of Japan as affected by salinity, drying and pH, with attention to their growth habitat. Botanica Marina 8: 199–217.
Olsen, Y.S., M. Sánchez-Camacho, N. Marbà, and C.M. Duarte. 2012. Mediterranean seagrass growth and demography responses to experimental warming. Estuaries and Coasts 35: 1205–1213.
Olsen, J.L., P. Rouzé, B. Verhelst, Y.-C. Lin, T. Bayer, J. Collen, E. Dattolo, E. De Paoli, S. Dittami, F. Maumus, G. Michel, A. Kersting, C. Lauritano, R. Lohaus, M. Töpel, T. Tonon, K. Vanneste, M. Amirebrahimi, J. Brakel, C. Boström, M. Chovatia, J. Grimwood, J.W. Jenkins, A. Jueterbock, A. Mraz, W.T. Stam, H. Tice, E. Bornberg-Bauer, P.J. Green, G.A. Pearson, G. Procaccini, C.M. Duarte, J. Schmutz, T.B.H. Reusch, and Y. Van de Peer. 2016. The genome of the seagrass Zostera marina reveals angiosperm adaptation to the sea. Nature 530: 331–335. doi:10.1038/nature16548.
Palomar, P., and I.J. Losada. 2010. Desalination in Spain: recent developments and recommendations. Desalination 225: 97–106.
Pérez-Ruzafa, A., C. Marcos, and J. Ros. 1991. Environmental and biological changes related to recent human activities in the Mar Menor. Marine Pollution Bulletin 23: 747–751.
Pergent, G., A. Djellouli, A.A. Hamza, K.S. Ettayeb, A.A. El Mansouri, F.M. Talha, M.A. Hamza, C. Pergent-Martin, and F. Platini. 2002. Characterization of the benthic vegetation in the Farwà Lagoon (Libya). Journal of Coastal Conservation 8: 119–126.
Ruiz, J.M., and J. Romero. 2001. Effects of in situ experimental shading on the Mediterranean seagrass Posidonia oceanica. Marine Ecology Progress Series 215: 107–120.
Ruiz, J.M., L. Marín-Guirao, and J.M. Sandoval-Gil. 2009a. Responses of the Mediterranean seagrass Posidonia oceanica to in situ simulated salinity increase. Botanica Marina 52: 459–470.
Ruiz, J.M., C.F. Boudouresque, and S. Enríquez. 2009b. Mediterranean seagrasses. Botanica Marina 52: 369–381.
Ruiz, J.M., C. Marcos, and J.L. Sánchez-Lizaso. 2010. Remote influence of off-shore fish farm waste on Mediterranean seagrass (Posidonia oceanica) meadow. Marine Environmental Research 69: 118–126.
Ruiz, J.M., J. Bernardeau, R. García Muñoz, and A. Ramos Segura. 2015. Monitoring network of Posidonia oceanica meadows and climate change in the region of Murcia: period 2004–2015. Seagrass Ecology Group, Spanish Institute of Oceanography, Oceanography Center of Murcia, Murcia, Spain, 152 pp. http://hdl.handle.net/10508/10139
Sánchez-Lizaso, J.L., J. Romero, J. Ruiz, E. Gacia, J.L. Buceta, O. Invers, Y. Fernández-Tormenada, J. Mas, A. Ruíz-Mateo, and M. Manzanera. 2008. Salinity tolerance of the Mediterranean seagrass Posidonia oceanica: recommendations to minimise the impact of brine discharges from desalination plants. Desalination 221: 602–607.
Sandoval-Gil, J.M., L. Marín-Guirao, and J.M. Ruiz. 2012a. Water relations and osmolyte concentrations in Mediterranean seagrasses (Posidonia oceanica and Cymodocea nodosa) in response to simulated salinity increase. Marine Biology 159: 1129–1141.
Sandoval-Gil, J.M., L. Marín-Guirao, and J.M. Ruiz. 2012b. Effect of salinity increase on photosynthesis, growth and survival of the seagrass Cymodocea nodosa. Estuarine, Coastal and Shelf Science 115: 260–271.
Sandoval-Gil, J.M., J.M. Ruiz, L. Marín-Guirao, J. Bernardeau-Esteller, and J.L. Sánchez-Lizaso. 2014a. Ecophysiological plasticity of shallow and deep populations of the Mediterranean seagrasses Posidonia oceanica and Cymodocea nodosa in response to hypersaline stress. Marine Environmental Research 95: 39–61.
Sandoval-Gil, J.M., I. Barrote, J. Silva, I. Olivé, M.M. Costa, J.M. Ruiz, L. Marín-Guirao, J.L. Sánchez-Lizaso, and R. Santos. 2014b. Plant-water relations of intertidal and subtidal seagrasses. Marine Ecology. doi:10.1111/maec.12230.
Schiermeier, Q. 2008. Water: purification with a pinch of salt. Nature 452: 260–261.
Serrano, O., M.A. Mateo, and P. Renom. 2011. Seasonal response of Posidonia oceanica to light disturbances. Marine Ecology Progress Series 423: 29–38.
Shibata, K. 1959. Spectrophotometry of translucence biological materials e opal glass transmission method. Methods of Biochemical Analysis 7: 77–109.
Short, F.T., and H. Neckles. 1999. The effects of global climate change on seagrasses. Aquatic Botany 63: 169–196.
Terrados, J., and J. Ros. 1991. Production dynamics in a macrophyte-dominated ecosystem: the Mar Menor coastal lagoon (SE Spain). Oecologia Aquatica 10: 255–270.
Tomasello, A., G. Di Maida, S. Calvo, M. Pirrota, M. Borra, and G. Procaccini. 2009. Seagrass meadows at the extreme of environmental tolerance: the case of Posidonia oceanica in a semi-enclosed coastal lagoon. Marine Ecology 30: 288–300.
Touchette, B.W. 2007. Seagrass-salinity interactions: physiological mechanisms used by submersed marine angiosperms for a life at sea. Journal of Experimental Marine Biology and Ecology 350: 194–215.
Touchette, B.W., and J.M. Burkholder. 2007. Carbon and nitrogen metabolism in the seagrass, Zostera marina L.: environmental control of enzymes involved in carbon allocation and nitrogen assimilation. Journal of Experimental Marine Biology and Ecology 350: 216–233.
Tyerman SD. 1982. Stationary Volumentric elastic modulus and osmotic pressure of the leaf cells of Halophilla ovalis, Zostera capricornii and Posidonia australis. Plant Physiology 69: 957–965.
Tyerman SD, Hatcher AI, West RJ, Larkum AWD. 1984. Posidonia australis growing in altered salinities: leaf growth, regulation of turgor and the development of osmotic gradients. Australian Journal of Plant Physiology 11: 35–47.
Tyerman, S.D. 1989. Solute and water relations of seagrasses. In Biology of Seagrasses: A treatise on the Biology of Seagrasses with special reference to the Australian region, ed. A.W.D. Larkum, A.J. Mc Comb, and S.A. Sheperd, 723–759. Amsterdam: Elsevier.
Velasco, J., J. Lloret, A. Millán, A. Marín, J. Barahona, P. Abellán, and D. Sánchez-Fernández. 2006. Nutrient and particulate inputs into the Mar Menor lagoon (SE Spain) from an intensive agricultural watershed. Water, Air and Soil Pollution 176: 37–56.
Vermaat, J.E., F.C.A. Verhagen, and D. Lindenburg. 2000. Contrasting responses in two populations of Zostera noltii Hornem. To experimental photoperiod manipulation at two salinities. Aquatic Botany 67: 179–189.
Verslues, P.E., M. Agarwal, S. Katiyar-Agarwal, J. Zhu, and J.K. Zhu. 2006. Methods and concepts in quantifying resistance to drought, salt and freezing, abiotic stresses that affect plant water status. Plant Journal 45: 523–539.
Villazán, B., T. Salo, F.G. Brun, J.J. Vergara, and M.F. Pedersen. 2015. High ammonium availability amplifies the adverse effect of low salinity on eelgrass Zostera marina. Marine Ecology Progress Series 536: 149–162.
Vizzini, S., G. Sarà, M.A. Mateo, and A. Mazzola. 2003. δ13C and δ15N variability in Posidonia oceanica associated with seasonality and plant fraction. Aquatic Botany 76: 195–202.
Walker, D.I., and A.J. McComb. 1990. Salinity response of the seagrass Amphibolis antarctica (Labill.) Sonder et Aschers: an experimental validation of field results. Aquatic Botany 36: 359–366.
Zar, J.H. 1984. Statistical significance of mutation frequencies, and the power of statistical testing using the Poisson distribution. Biometrical Journal 26: 83–88.
Zieman, J.C. 1974. Methods for the study of the growth and production of turtlegrass Thalassia testudinum König. Aquaculture 4: 139–143.
Acknowledgements
This research was funded by the Ministry of Science and Innovation of the Spanish Government through the project OSMOGRASS II (project ref. CTM2009-08413MAR) and RECCAM (project ref. CTM2013-48027-C3-2-R). During the writing of the paper, L. Marín-Guirao was supported by a Marie-Curie Fellowship (FP7-PEOPLE-IEF-2013; HEATGRASS Project). We express our gratitude to Arantxa Ramos-Segura for their field and laboratory support. This study was also supported by the project Monitoring network of P. oceanica meadows and global climate change of the Murcia Region funded by the Autonomous Government of the Murcia Region (General Directorate of Livestock and Fishery) and the European Fishery Fund (EFF 2007–2013). We would also like to thank the General Directorate of Fishery Resources and Aquaculture of the Spanish Ministry of the Environment and the General Directorate of the Environment of the Regional Government for their support in the field sampling performed in the declared Zone of Special Bird Protection Isla Grosa (ZEPA ES0000200) of the Natura 2000 Network.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by Ken Dunton
Rights and permissions
About this article
Cite this article
Marín-Guirao, L., Sandoval-Gil, J.M., García-Muñoz, R. et al. The Stenohaline Seagrass Posidonia oceanica Can Persist in Natural Environments Under Fluctuating Hypersaline Conditions. Estuaries and Coasts 40, 1688–1704 (2017). https://doi.org/10.1007/s12237-017-0242-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12237-017-0242-1